PRE-EXPOSURE PROPHYLAXIS - update January 2023
PrEP or PPrE
What is PrEP?
Pre-exposure prophylaxis for HIV (PrEP), is a preventive treatment with specific antiretroviral drugs for HIV. These drugs have been used since the late 1990s in the treatment of HIV. Once used in HIV-negative individuals, this preventive treatment greatly reduces the risk of HIV infection. PrEP reduces the possibility of contracting HIV following a risky relationship. However, it does not completely eliminate the risk of HIV infection. PrEP could be called the 'contraceptive pill' against HIV.
What molecules are used in PrEP?
The only molecules currently approved in Canada for PrEP are Truvada or its generic equivalent tenofovir disoproxil fumarate (TDF)/ emtricitabine (FTC) 300mg/200mg and Descovy or tenofovir alafenamide (TAF) 25mg/emtricitabine (FTC) 200mg without a generic equivalent currently. Both medications are taken orally.
The World Health Organization (WHO) recommends the use of injectable cabotegravir in men and women, as well as the dapivirine ring. The dapivirine ring is unlikely to be recommended in Canada due to its low efficacy.
The 3 studies on the dapivirine ring yield weak results:
Ring study, overall risk reduction of 35%.
ASPIRE study, overall risk reduction of 27%.
HOPE (HIV Open-label Prevention Extension) study and DREAM (Dapivirine Ring Extended Access and Monitoring) study show a greater risk reduction of over 50%.
What is the effectiveness of PrEP?
The effectiveness of PrEP is strictly linked to its adherence. For PrEP to be effective, it must be taken regularly. According to various studies, the effectiveness of PrEP can go up to 100% according to the iPrEx Ole study.
Other studies show high effectiveness if taken diligently:
Partners PrEP - continuous, heterosexual relationships, men and women, effectiveness depending on the level of detected drugs 82%.
PROUD - continuous, HARSAH and transgender women, effectiveness depending on the level of detected drugs 86%.
IPERGAY - on-demand, HARSAH and transgender women, effectiveness according to pill count 86%.
IPERGAY Open Label Extension - on-demand, HARSAH and transgender women, effectiveness 97%.
DISCOVER - continuous, HARSAH, transgender women, effectiveness depending on the level of detected drugs 99%.
PRÉVENIR - continuous and on-demand, 50-50, HARSAH and transgender women, 3 infections in each arm, no difference in effectiveness between continuous and on-demand use, effectiveness 99%.
HTPN 083 - injectable, HARSAH and transgender women, study terminated early due to its superiority over TDF/FTC (generic Truvada), overall efficacy not adjusted for drug adherence 66%.
HTPN 084 - injectable cisgender women, results pending.
Continuous or on-demand PrEP?
In Canada, only continuous PrEP is officially approved and recommended. However, we have 4 studies, IPERGAY, IPERGAY Open Label Extension, PRÉVENIR, and PROTÈGES, which demonstrate high effectiveness. The PRÉVENIR and PROTÈGES studies show no difference in HIV infections between on-demand and continuous use. The only molecule officially recommended for on-demand use is TDF/FTC (Truvada or its generic). We currently have no clinical studies showing the possibility of using Descovy (TAF/FTC) on-demand. However, based on pharmacokinetics, Descovy (TAF/FTC) should have the same effectiveness on-demand as TDF/FTC (Truvada or its generic).
In general, if a person has at least one sexual relationship per week and this relationship is not anticipated, continuous use should be favored. If relationships are anticipated or sporadic, on-demand use may be adequate.
When traveling, only continuous PrEP should be considered since relationships are rarely anticipated or planned. With on-demand PrEP, you must wait 2 hours after taking 2 TDF/FTC tablets for protection to be present. Adventures during travel happen 'here and now,' making on-demand use potentially ineffective and should be discouraged.
What is the difference between Truvada (TDF/FTC 300mg/200mg) and Descovy (TAF/FTC 25mg/200mg)?
Both medications contain the same active molecules which are tenofovir (TDF or TAF) and emtricitabine (FTC). The main difference is in the absorption of tenofovir by cells and the blood losses of tenofovir. TDF (tenofovir disoproxil fumarate) contains 300mg of tenofovir. Only 10% to 20% of the drug is absorbed and the rest is lost in the bloodstream. Since tenofovir is eliminated by the kidneys, the high blood concentration of tenofovir can overload the kidneys and cause proximal renal tubular acidosis also known as Fanconi syndrome. In short, the kidneys become overloaded and tenofovir prevents the reabsorption of various molecules or minerals in the renal tubules causing loss of various molecules and minerals including phosphorus. The natural reservoir of phosphorus is in our bones. When our body detects a phosphorus deficiency, it reabsorbs it from our bones causing demineralization and bone fragility (osteopenia / osteoporosis). Descovy (TAF/FTC) does not cause the same problems. In the Descovy tablet, there is only 25mg of tenofovir in the form of TAF (tenofovir alafenamide) so 90% is absorbed by cells giving about 10% of blood losses.
Other differences between the two products are:
Truvada or TDF/FTC should not be given to people with decreased kidney function (less than 50mL/min) or to those with underlying bone fragility. TDF/FTC was studied and is indicated for continuous use in men and women, cis and trans. TDF/FTC is also indicated for on-demand use. On-demand use is only indicated in cisgender men and transgender women. On-demand use does not offer protection in cisgender women and should not be administered in this way.
The use of PrEP in people with chronic hepatitis B should always be discussed. Intermittent (on-demand) use is contraindicated. Only continuous use should be offered, explaining to the patient that stopping tenofovir could cause symptoms of acute hepatitis B to reappear. Intermittent use risks making the virus resistant to tenofovir. Currently, tenofovir is a powerful remedy for treating this infection. Developing resistance to this drug could limit therapeutic options available on the market due to the risk of cross-resistance.
Descovy or TAF/FTC should be used with caution in people with underlying hypercholesterolemia. There are studies suggesting potential weight gain with TAF/FTC. It is unlikely that Descovy causes kidney problems or bone demineralization. Descovy is not yet indicated for PrEP in women. Its use was only studied for continuous use. There is no data yet for on-demand use. According to the pharmacokinetics of TAF, its intermittent use should be as effective as with TDF. Descovy should not be used in people taking certain anti-epileptics that decrease the concentration of TAF and could render protection ineffective.
Truvada (TDF/FTC) and Descovy (TAF/FTC) do not interact with alcohol or recreational substances. Their use is not contraindicated although using both increases the risk of poor adherence, extreme behaviors, and physical as well as mental health problems (toxic psychosis also known as a bad trip).
Does PrEP offer absolute protection against HIV?
No, PrEP does not provide absolute protection. If a person has contact with an HIV strain resistant to the molecules used for PrEP, protection may be diminished or ineffective. There have been a few documented cases of HIV transmission despite PrEP being taken correctly with resistant strains: the case of Dr. J. Fox (BHIVA 2015), the case of Dr. David Knox (CROI 2016), the case of Dr. Howard Grossman (HIVR4P 2016), and the case of the HTPN 083 study. However, the risk of HIV transmission with a resistant strain remains very low.
Can I skip PrEP doses?
No, PrEP doses should not be skipped. Several studies have shown substantial PrEP failure if the medication is not taken regularly. Poor adherence to preventive treatment with PrEP in the VOICE, FEM-PrEP, and ASPIRE studies demonstrated the importance of regular medication intake. Taking PrEP as prescribed by the healthcare professional is crucial to achieving adequate protection against HIV.
Does PrEP protect against other STIs or pregnancy?
No, PrEP does not protect against other sexually and blood-transmitted infections (STIs) such as, for example, gonorrhea, chlamydia, syphilis, or hepatitis C.
Who should be offered PrEP?
PrEP is intended for HIV-negative individuals who have a substantial risk of contracting HIV, who have good kidney function (creatinine clearance greater than 50mL/min) for Truvada (TDF/FTC) and 30mL/min for Descovy. Previously, PrEP was recommended only for individuals who were part of one of the vulnerable groups for having high-risk sexual relationships for HIV:
- men who have sex with men (HARSAH)
- transgender women
- sex workers
- intravenous and/or intranasal drug users with risk of sharing equipment and contact with HIV
- serodiscordant couples for HIV where the HIV-positive partner has a detectable viral load (more than 40 copies/mL) or is not adherent to HIV treatment
- serodiscordant couples for HIV where the HIV-positive partner has just started HIV treatment. PrEP in this case should be used for 6 months or until the HIV-positive partner becomes undetectable (viral load less than 40 copies/mL)
According to the new CDC (Center of Disease Control) update from 2021, PrEP should be given to any individual desiring to start it after discussion.
Who should not take PrEP?
- Anyone already living with HIV
- HIV-negative individuals who remain in a monogamous relationship with an HIV-positive partner who is treated, adherent to treatment, and undetectable (VL less than 40 copies/mL) for more than 6 months without other sexually transmitted infections (STIs)
- Anyone who consistently practices safe sex without taking risks
- Anyone presenting an allergic reaction to a component of the drug
With whom should the risks and benefits be discussed before offering PrEP?
- Anyone with decreased kidney function (creatinine clearance less than 60 mL/min) to avoid worsening the renal condition.
- Anyone requiring long-term treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) such as naproxen (Naprosyn), celecoxib (Celebrex), ibuprofen (Advil, Motrin), diclofenac (component of Artrotec), or others since NSAIDs could potentially increase the risk of kidney damage if taken concurrently with TDF/FTC.
- Anyone living with hepatitis B virus should discuss the risks and benefits with their doctor before starting PrEP. TDF in PrEP is also one of the treatments for chronic hepatitis B virus. Once a person living with hepatitis B virus starts PrEP, they will need to take it for a very long time, even for life. If PrEP is taken irregularly, the hepatitis B virus may become resistant to TDF, a powerful treatment against this infection. If the person decides to stop or suspend their PrEP treatment, they risk developing symptoms of acute hepatitis B infection. Additionally, a person living with hepatitis B virus should never take PrEP on demand but rather continuously.
- Anyone known for osteopenia or osteoporosis (decreased bone density) or who has had pathological fractures (fragility). TDF/FTC could worsen these conditions by further decreasing bone density.
- Anyone with significant or morbid obesity. TDF/FTC could worsen liver hepatotoxicity that is already steatotic (toxicity of the liver that is fatty) and cause lactic acidosis (acid-base imbalance) especially in very obese women. Lactic acidosis is a medical emergency that can have very serious consequences including death.
What constitutes a high-risk sexual relationship?
Any anal or vaginal intercourse without a condom regardless of the duration of unprotected penetration (a few seconds or a few quarters of an hour) with a person considered at risk for HIV (HARSAH, drug users, person from HIV-endemic areas)
The risk of HIV transmission via anal intercourse per sexual act
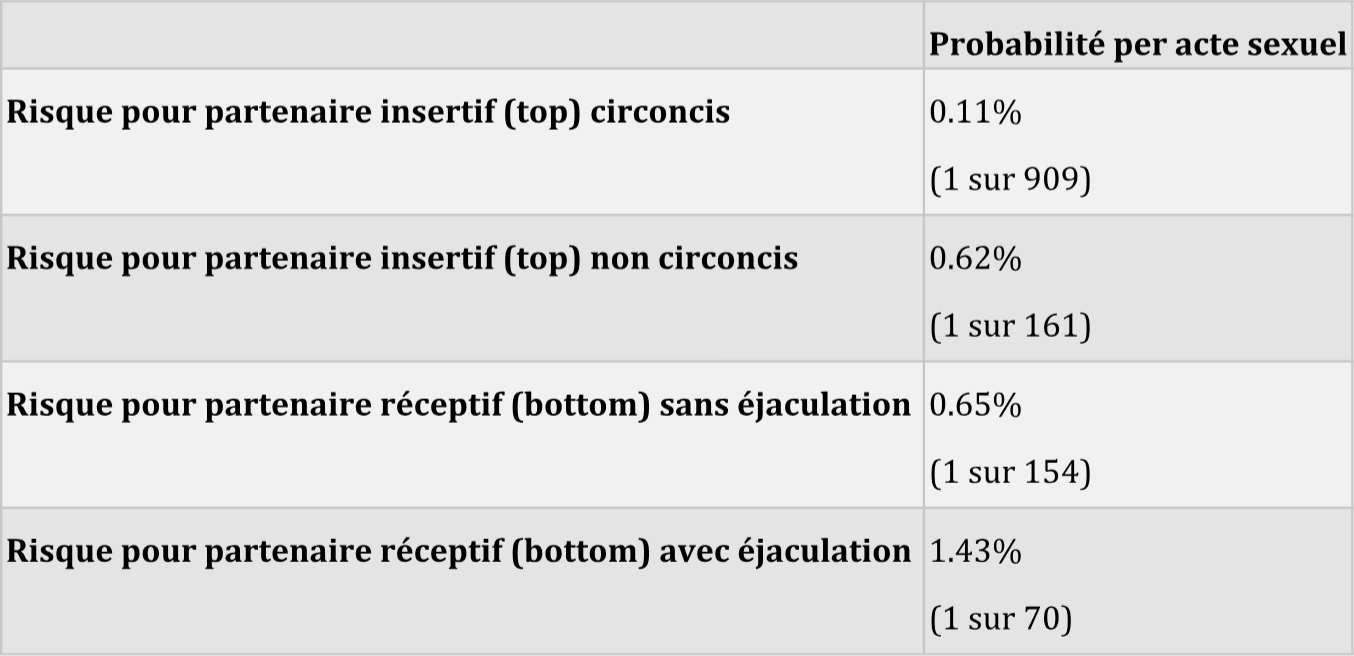
Source: Jin F et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART.
AIDS, Published online ahead of print, 2010.
Is oral sex or 'fingering' someone also risky for HIV?
As for oral sex, cunnilingus (mouth-vagina), anilingus (mouth-anus), and insertive fellatio (putting the penis in the other person's mouth) carry a theoretical risk of HIV transmission (highly unlikely) with no documented cases in the literature.
As for receptive fellatio (taking the penis in the mouth), the risk of HIV transmission is more than theoretical, especially if ejaculation occurs in the mouth with ulcers or abrasions in the oral cavity post dental procedures (tooth brushing or flossing). However, there is no definitive evidence regarding these factors. The risk of oral HIV transmission through insertive fellatio remains lower than all other forms of sexual intercourse. The low risk of HIV transmission through insertive fellatio is contributed to by the inhibitory activity of HIV by salivary enzymes, the low salt content in saliva, a lower susceptibility to HIV of the tissue of the oral cavity, and the virucidal activity of digestive enzymes in the stomach.
Fingering someone carries only a theoretical risk (highly unlikely) even if there are skin abrasions. No cases have been documented of this form of transmission in the literature.
What medication is used for PrEP?
The only approved medication currently used for PrEP is tenofovir disoproxil fumarate (TDF) together with emtricitabine (FTC) also known by the brand name Truvada.
Other molecules are currently being studied for PrEP use such as tenofovir alafenamide (TAF) together with emtricitabine (FTC) known by the brand name Descovy. This molecule is currently being studied in the Discover study and demonstrates very good protection against HIV according to preliminary data. The advantage of this molecule is taking the medication with decreased creatinine clearance even up to 30 milliliters per minute. Descovy also presents a lower risk of renal or bone side effects than TDF/FTC.
Other molecules like maraviroc, intravaginal tenofovir (CAPRISA study), or dapivirine vaginal ring (ASPIRE study) are also being studied.
What are the side effects and/or serious effects of taking PrEP?
Short-term side effects may include nausea, vomiting, abdominal cramps, dizziness, headaches, and fatigue. These symptoms usually start within the first two weeks of taking TDF/FTC and last only one to two weeks. Often taking the medication with food will decrease gastrointestinal symptoms. Taking TDF/FTC at bedtime reduces fatigue symptoms.
Long-term and serious side effects include proximal renal tubular acidosis (Fanconi syndrome) and bone demineralization (osteoporosis/osteopenia).
Fanconi syndrome is characterized by a reabsorption defect with urinary leakage of proteins, glucose, phosphate, amino acids, bicarbonates, and other organic compounds. It occurs in less than 5 percent of cases and is mostly reversible by stopping TDF/FTC. There have been very rare cases of severe kidney failure requiring kidney transplants.
The risk of Fanconi syndrome may be increased in
- elderly individuals
- individuals of black or Asian race
- indigenous people
- obese individuals
- smokers
- individuals with diabetes mellitus
- individuals coinfected with hepatitis C virus
- individuals with high blood pressure, dyslipidemia, or cardiovascular disease
- individuals with kidney stones or kidney infections
- individuals with sickle cell anemia
- individuals living with autoimmune conditions such as lupus, for example
- individuals with a family history of kidney disease
The risk of osteoporosis or osteopenia may be increased in
- women
- elderly individuals
- individuals of white or Asian race
- individuals with a family history of osteoporosis or fragility fractures
- underweight individuals or those suffering from anorexia
- individuals with hormonal imbalances such as hypothyroidism, hypotestosteronemia (low testosterone), or hypoestrogenemia (low estrogen)
- smokers, alcohol abusers, those on methadone/opiates, or physically inactive individuals
- individuals with vitamin D and/or calcium deficiency
- individuals on long-term cortisone therapy
- individuals living with HIV, diabetes mellitus, chronic infections, chronic kidney disease, or taking certain medications such as anticoagulants, anticonvulsants, antipsychotics, cyclosporines, glitazones, proton pump inhibitors, or methotrexate.
What are the drug interactions of PrEP with other medications?
There are no significant interactions of PrEP with other medications except for some treatments for hepatitis C virus and SGLT2 inhibitors in the treatment of diabetes.
FTC may have an interaction with orlistat (weight loss medication) by altering the absorption of orlistat.
TDF may increase the risk of nephrotoxicity if combined with SGLT2 inhibitors like canagliflozin (Invokana), dapagliflozin (ForxigaTM), empagliflozin (JardianceTM). Renal function should be closely monitored.
The Liverpool HIV drug interaction website allows checking interactions with other medications. The site is accessible at
How should PrEP be taken?
Continuous PrEP
TDF/FTC should be started 7 days before the first risky encounter for men and 21 days for women, taken daily, and if the decision to stop PrEP is made, it should be continued for at least 2 to 28 days after the last encounter.
Continuous PrEP is currently the most reliable method with multiple studies confirming its effectiveness up to 100% with good treatment adherence (studies IPREX, IPREX Ole, PROUD, partners PrEP, partners demonstration project, Bangkok TDF, TDF 2 and two ongoing studies currently DISCOVER and PROTÈGE)
Continuous PrEP should not be taken if the person requires long-term treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) such as naproxen (Naprosyn), celecoxib (Celebrex), ibuprofen (Advil, Motrin), diclofenac (component of Artrotec), or others.
On-Demand PrEP
2 tablets of TDF/FTC should be taken between 2 hours and 24 hours before sexual intercourse and 1 tablet should be taken on the first and second day after sexual intercourse.
This method was studied only in men who have sex with men (MSM). It is not recommended for women and users of intravenous or intranasal drugs.
On-Demand PrEP should not be taken by people living with chronic viral hepatitis B.
The limitation of this study is that it was tested by a single study called IPERGAY in France together with Quebec. The established efficacy of On-Demand PrEP was 86%. A second study called PROTÈGE also tests the effectiveness of this method; however, the final results are not yet available. Preliminary results seem very promising for On-Demand PrEP.
Holiday PrEP
Some people always protect themselves when they are at home but take risks when traveling. PrEP can be considered if short-term risks are anticipated.
Daily PrEP can be taken 7 days before the trip, throughout the trip, and 7 days after the trip. (Taking PrEP after the last risky sexual encounter should occur at least 2 days after and up to 28 days after). PrEP for 7 days before, throughout the trip, and 7 days after is an unpublished recommendation but in line with Quebec and Canadian recommendations.
Why use TDF/FTC in combination for PrEP?
The biological properties of FTC and TDF make them attractive first-generation agents for PrEP:
- Powerful antiretroviral activity against most HIV subtypes with a high resistance barrier
- Few cases of transmission of TDF/FTC-resistant strains
- Early activity in the HIV life cycle
- Long intracellular half-life, capable of reaching high concentrations in the genital tract
- Convenient once-daily dosing and few drug interactions
- Established safety profile based on their use in combination as antiretroviral therapy (ART)
What is the follow-up for PrEP?
PrEP follow-up should be done 1 month after starting pre-exposure prophylaxis treatment and then every three months with tests as suggested in Canadian and/or Quebec guidelines.
Canadian guidelines for PrEP and PEP
Read thedocumenton the official Canadian website.